Abstract
Introduction: Patients with hereditary hemochromatosis (HH) require continued phlebotomies to limit end-organ damage. Approximately 25% of patients in maintenance felt receiving phlebotomies was "inconvenient" or "very inconvenient" (Brisott et al, 2011). Patient compliance with phlebotomies generally declines with time (Hicken et al, 2003), and therapeutic phlebotomies may not be medically suitable for some HH patients. Rusfertide, a peptide mimetic of hepcidin, is an effective regulator of iron distribution and utilization that has demonstrated control of iron in an animal model of HH.
Methods: We conducted an open-label, dose-finding efficacy study that investigated subcutaneous rusfertide in HH patients on a stable phlebotomy regimen of 0.25 to 1 phlebotomy per month for at least 6 months. Patients with clinical laboratory abnormalities and those receiving iron chelation therapy or erythrocytapheresis were excluded. Subjects received individually titrated rusfertide doses once or twice a week to maintain transferrin saturation (TSAT) below 45% and were followed for 6 months. Study measures included TSAT, serum iron, transferrin and ferritin, liver iron concentration (LIC) measured by MRI, and adverse events (AEs).
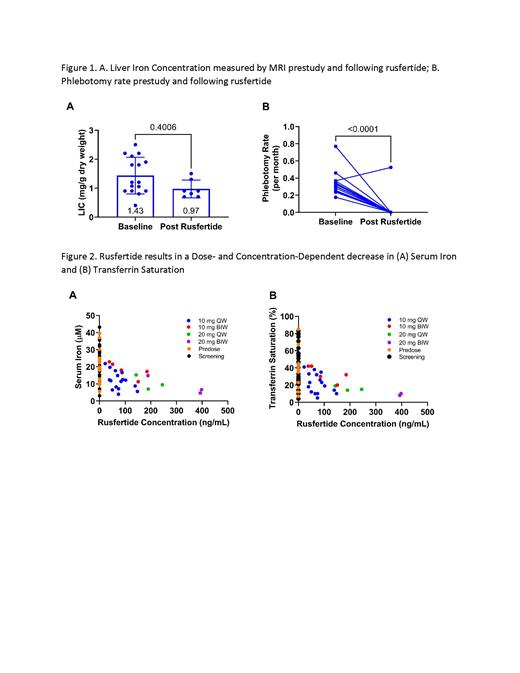
Results: Sixteen subjects (10 male/6 female) were enrolled. Mean age and weight were 62.5 years and 88.1 kg, respectively. LIC values were maintained at pre-study levels, with minimal use of phlebotomies during the duration of the study (Figure 1A). Average pre-study phlebotomy rate was 0.27 phlebotomies/month compared to 0.03 phlebotomies/month during the study (p<0.0001; Figure 1B). There was a dose- and concentration-dependent decrease in serum iron and TSAT (Figure 2A and 2B). Transferrin levels were relatively constant over the course of the study. There were no notable changes in hematological parameters such as hematocrit, erythrocytes, leucocytes, or platelets. Rusfertide was generally well tolerated. Adverse events reported in 2 or more subjects included diarrhea, fatigue, injection site reactions (erythema, induration, pain, pruritis), dizziness, and headache.
Conclusions: Rusfertide demonstrated a pharmacodynamic effect in reducing TSAT and serum iron, with corresponding significant reduction in the number of phlebotomies, and with LIC maintained at pre-study levels with minimal use of phlebotomies. These data indicate rusfertide was well tolerated in patients with HH and controls LIC, supporting development of rusfertide as a potential treatment for HH.
Kowdley: PTG: Consultancy, Research Funding. Modi: Protagonist Therapeutics: Current Employment. Valone: Protagonist Therapeutics: Current Employment, Current equity holder in publicly-traded company. Gupta: Protagonist Therapeutics: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal